
Resonance is a mental exercise and method within the Valence Bond Theory of bonding that describes the delocalization of electrons within molecules. It compares and contrasts two or more possible Lewis structures that can represent a particular molecule. Resonance structures are used when one Lewis structure for a single molecule cannot fully describe the bonding that takes place between neighboring atoms relative to the empirical data for the actual bond lengths between those atoms. The net sum of valid resonance structures is defined as a resonance hybrid, which represents the overall delocalization of electrons within the molecule. A molecule that has several resonance structures is more stable than one with fewer. Some resonance structures are more favorable than others.
Electrons have no fixed position in atoms, compounds and molecules (see image below) but have probabilities of being found in certain spaces (orbitals). Resonance forms illustrate areas of higher probabilities (electron densities). This is like holding your hat in either your right hand or your left. The term Resonance is applied when there are two or more possibilities available. Resonance structures do not change the relative positions of the atoms like your arms in the metaphor. The skeleton of the Lewis Structure remains the same, only the electron locations change.
“PICK THE CORRECT ARROW FOR THE JOB”
Most arrows in chemistry cannot be used interchangeably and care must be given to selecting the correct arrow for the job.

An animation of how one can do a resonance with ozone by moving electrons
In resonance structures, the electrons are able to move to help stabilize the molecule. This movement of the electrons is called delocalization.
Even though the structures look the same, the formal charge (FC) may not be. Formal charges are charges that are assigned to a specific atom in a molecule. If computed correctly, the overall formal charge of the molecule should be the same as the oxidation charge of the molecule (the charge when you write out the empirical and molecular formula). We want to choose the resonance structure with the least formal charges that add up to zero or the charge of the overall molecule. The equation for finding Formal Charge is:
Formal Charge = (number of valence electrons in free orbital) – (number of lone-pair electrons) – ( [latex] \frac [/latex] number bond pair electrons)
The formal charge has to equal the molecule’s overall charge,e.g., the [latex] CNS^- [/latex] has an overall charge of -1, so the Lewis structure’s formal charge has to equal -1.
Consider the thiocyanate ([latex] CNS^- [/latex]) ion.
1. Find the Lewis Structure of the molecule. (Remember the Lewis Structure rules.)

2. Resonance: All elements want an octet, and we can do that in multiple ways by moving the terminal atom’s electrons around (bonds too).

3. Assign Formal Charges
Formal Charge = (number of valence electrons in free orbital) – (number of lone-pair electrons) – ( [latex] \frac [/latex] number bond pair electrons)
Remember to determine the number of valence electron each atom has before assigning Formal Charges
C = 4 valence e – , N = 5 valence e – , S = 6 valence e – , also add an extra electron for the (-1) charge. The total of valence electrons is 16.

4. Find the most ideal resonance structure. (Note: It is the one with the least formal charges that adds up to zero or to the molecule’s overall charge.)

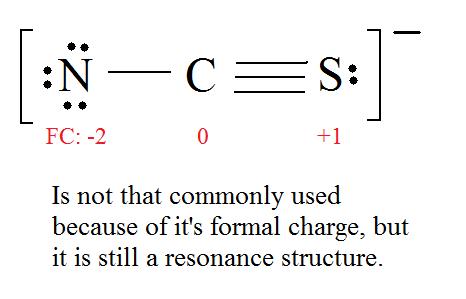
5. Now we have to look at electronegativity for the “Correct” Lewis structure.
The most electronegative atom usually has the negative formal charge, while the least electronegative atom usually has the positive formal charges.

Resonance Structures are a representation of a Resonance Hybrid, which is the combination of all resonance structures. The resonance structure with the Formal Charge closest to zero is the most accepted structure, however, the correct Lewis structure is actually a combination of all the resonance structures and is not solely describe as one.
Note: The correct Lewis structure is actually a combination of all the resonance structures and hence is not solely described as one.
Consider the carbonate ion: CO3 2 –

Step 1: Draw the Lewis Structure & Resonance.

Step 2: Combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed.

Step 3: Add only the lone pairs found on ALL resonance structures.

The bottom is the finished resonance hybrid for CO3 2- .
Benzene is an extremely stable molecule and it is accounted for its geometry and molecular orbital interaction, but most importantly it’s due to its resonance structures. The delocalized electrons in the benzene ring make the molecule very stable and with its characteristics of a nucleophile, it will react with a strong electrophile only and after the first reactivity, the substituted benzene will depend on its resonance to direct the next position for the reaction to add a second substituent.

Aminophenol is a very stable molecule that is present in most biological systems, mainly in proteins. By studies of NMR spectroscopy and X-Ray crystallography it is confirmed that the stability of the amide is due to resonance which through molecular orbital interaction creates almost a double bond between the Nitrogen and the carbon.

Molecules with multiple resonance forms


Some structural resonance conformations are the major contributor or the dominant forms that the molecule exists. For example, if we look at the above rules for estimating the stability of a molecule, we see that for the third molecule the first and second forms are the major contributors for the overall stability of the molecule. The nitrogen is more electronegative than carbon so, it can handle the negative charge more than carbon. A carbon with a negative charge is the least favorable conformation for the molecule to exist, so the last resonance form contributes very little for the stability of the Ion.
The Hybrid Resonance forms show the different Lewis structures with the electron been delocalized. This is very important for the reactivity of chlorobenzene because in the presence of an electrophile it will react and the formation of another bond will be directed and determine by resonance. The long pair of electrons delocalized in the aromatic substituted ring is where it can potentially form a new bond with an electrophile, as it is shown there are three possible places that reactivity can take place, the first to react will take place at the para position with respect to the chloro substituent and then to either ortho position.



1. False, because the electrons were not moved around, only the atoms (this violates the Resonance Structure Rules).
2. Below are the all Lewis dot structure with formal charges (in red) for Sulfate (SO4 2 – ). There isn’t a most favorable resonance of the Sulfate ion because they are all identical in charge and there is no change in Electronegativity between the Oxygen atoms.

3. Below is the resonance for CH3COO – , formal charges are displayed in red. The Lewis Structure with the most formal charges is not desirable, because we want the Lewis Structure with the least formal charge.


4. The resonance for HPO3 2 – , and the formal charges (in red).
5. The resonance for CHO2 1 – , and the formal charges (in red).

6. The resonance hybrid for PO4 3 – , hybrid bonds are in red.

7. The resonance hybrid for NO3 – , hybrid bonds are in red.

Problems #2